Research
Our group’s research program applies computational methods to fundamental and practical problems at the chemical, physical, biological, and material sciences interface. We are working towards developing novel design strategies for metal-based catalysts, sensors, and materials with excellent optoelectronic properties materials in different areas of science like nanotechnology, catalysis, atmospheric chemistry, optics, materials physics, biophysics etc. With the aid of theoretical techniques, we also aim to reveal and understand the microscopic mechanisms of poorly understand chemical and photochemical processes in complex chemical, physical and biological systems.
Our research spans over four broad areas :Machine Learning Applications in Catalysis, Mechanistic study of various class of chemical reactions which involves atmospheric reactions, and catalytic reactions, Design strategy for Efficient chromophores for Organic Photovoltaics, and Design principles for Localized surface plasmon resonance (LSPR) nanostructures for sensing and solar cell applications.
Here are some brief write-ups on the themes and topics that we currently work on:
Machine Learning Applications in Catalysis
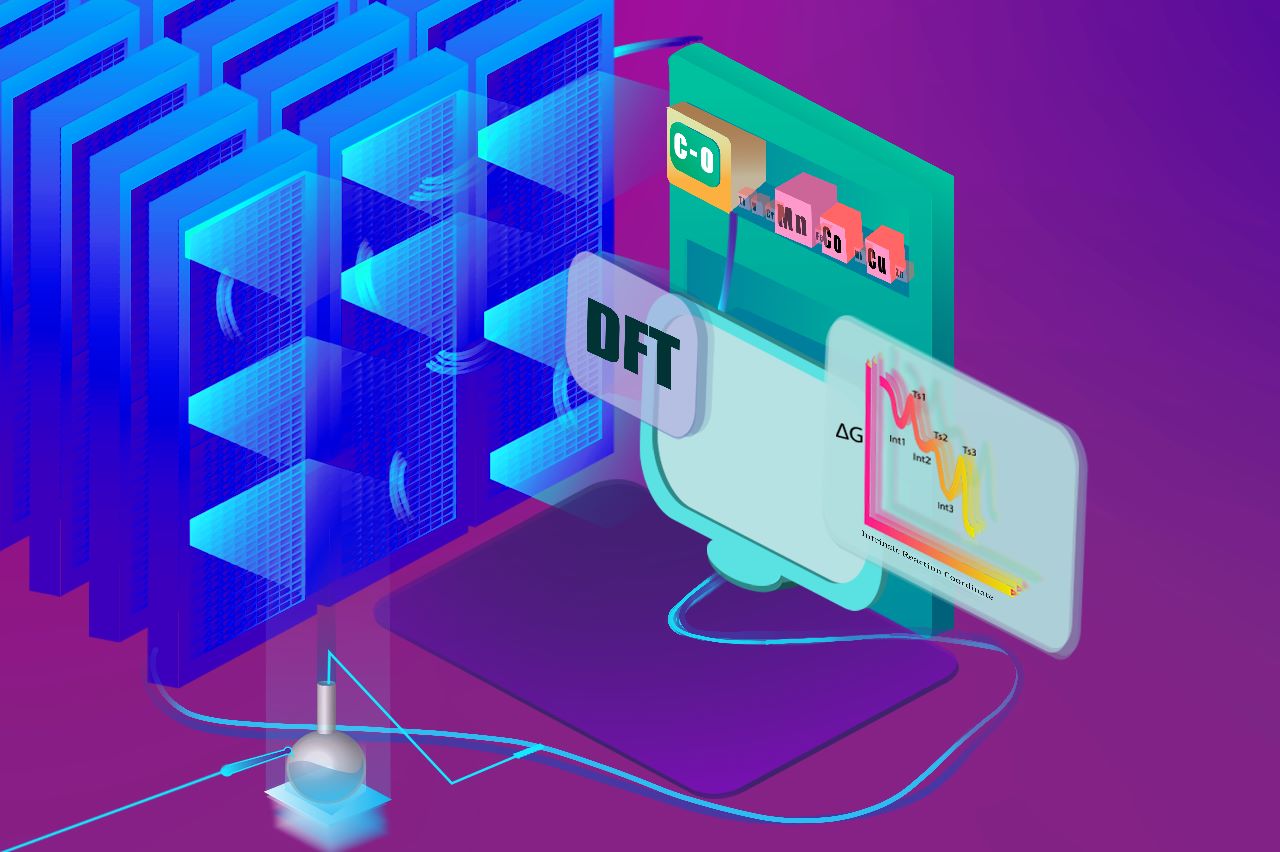
In the realm of catalysis, the integration of machine learning techniques has emerged as a transformative force, revolutionizing our understanding and optimization of catalytic processes. By leveraging the power of data-driven insights, we are pushing the boundaries of catalyst design, ultimately contributing to the sustainable development of cleaner and more efficient chemical processes. Our ongoing work in this dynamic field continues to unravel new possibilities and reshape the landscape of catalytic research.
By employing machine learning algorithms, we are developing predictive models for predicting the reaction outcomes of various transition metal-catalyzed organic reactions. These models leverage diverse data sources, including experimental results, quantum mechanical calculations, and structural information, allowing us to rapidly assess and prioritize potential catalysts for targeted reactions. Further, these ML algorithms are also used for the elucidation of complex reaction mechanisms by analyzing experimental and computational data. This has facilitated a deeper understanding of catalytic processes, aiding in the identification of key intermediates and pathways, thereby guiding the rational design of catalysts with improved performance. This data-driven approach allows for the identification of optimal conditions that might be challenging to deduce through traditional experimental design. </div>
Localized surface plasmon resonace (LSPR) nanostructures for sensing and solar cell applications
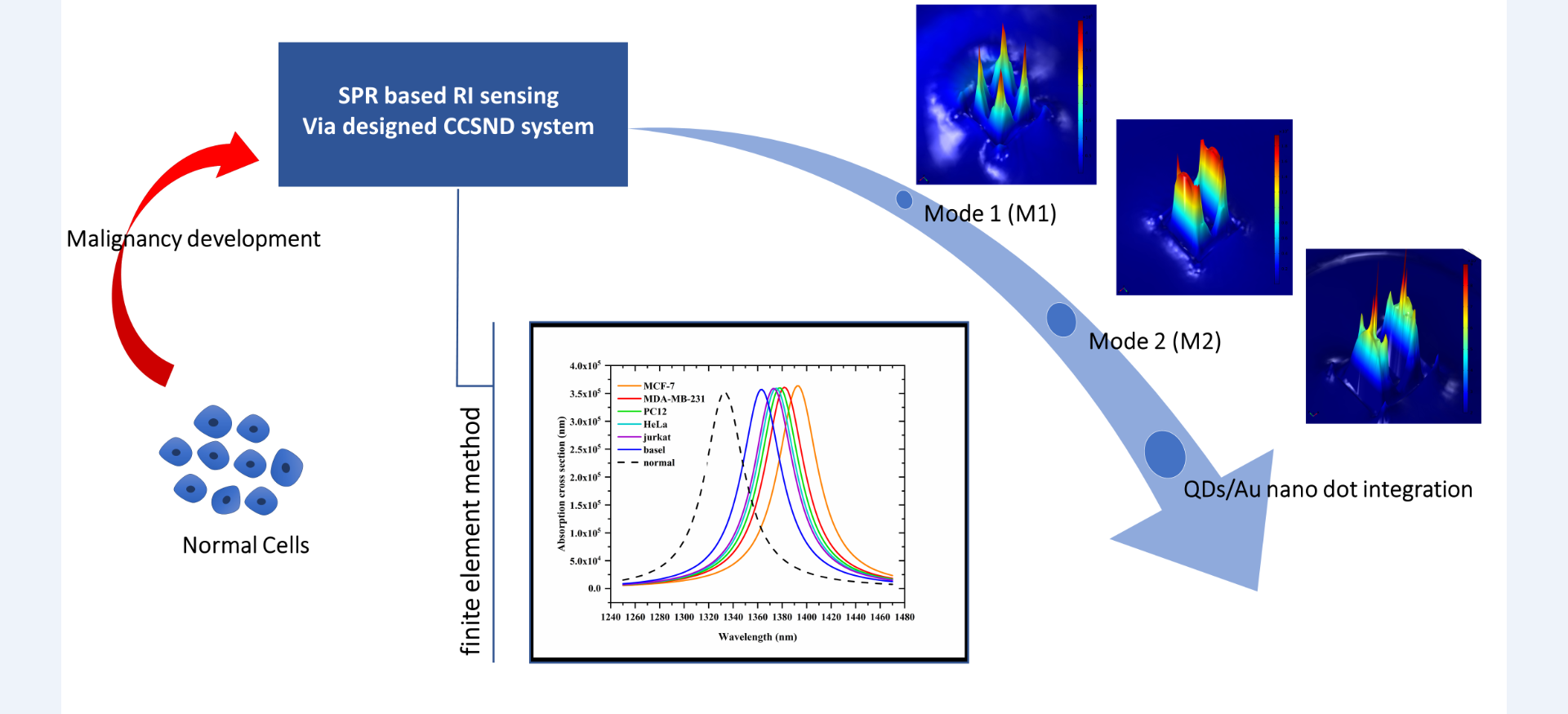
Following its broad applicability in chemical and biosensing, the characteristic light-matter interaction at the nanoscale regime exhibited by plasmonic structures has recently sparked a lot of interest. The plasmonics wing at the VIT group in collaboration with Dr. Jesly Jacob focuses on localized surface plasmon resonance (LSPR) refractive index sensors that take advantage of the highly tuneable resonant profiles of plasmonic structures, specifically cavity-based nanoplatforms. Theoretical simulations and performance optimizations are carried out via finite element method. Fine-tuning of the geometrical and material parameters of nanomaterials resulted in proposing hybrid nano sensor platforms with improved performance parameters. Quantum dots, metallic nano dots, nanowires etc. following their plasmon- exciton and dual plasmon coupling are qualitatively exploited for productive structure performance. In addition, breaking of symmetry, channel based multi analyte sensing and direction dependent functionality forms the currently ongoing research topics.
Mechanistic Exploration of Transition Metal Catalyzed Cross-Coupling Reactions using Quantum Chemical Studies
The significance of cross-coupling reactions in the arena of chemistry, could be delineated by the Nobel prize in 2010 for Pd catalyzed cross-coupling reactions. These reactions have got a wide range of applications both in academia and industry. The coupling of theoretical methods with experimental techniques could avail to iron out the dilemmas of the complex reactions and helps to rationalize the experimental observations. Our work is primarily based on the cross-coupling reactions catalyzed by earth abundant, economical, first-row late transition metals like Fe, Co, Ni, Cu and Zn. Due consideration is given to both carbon-carbon and carbon-heteroatom cross coupling reactions. Using the Density Functional Theory (DFT) methods, the thermodynamics and kinetics of the key steps involved in the cross-coupling reactions could be explored. Finding out the most feasible pathway for a particular reaction, could be evoked by probing the nature of transition states, involved in the crucial steps. Due to the inadequacy of experimental techniques in characterizing the transition states, computational techniques emerged as an excellent tool to probe the details of the former. We diligently collaborate with the experimental catalysis research group of Dr. G. Anilkumar at School of Chemical sciences, MGU, Kerala. For good measure, we also explore the metal- ligand interactions of the active catalytic species present in the reaction. Insights gained in this way will aid in the designing of efficient catalysts and could be extended further to the design of biologically active compounds like benzofuran derivatives.
Efficient chromophores for Organic Photovoltaics
Organic Solar Cells are considered as a promising photovoltaic technology which has the potential to provide moderate power conversion efficiencies with low cost and easy processability. Organic solar cells utilises organic molecules as chromophores which absorbs light and generates excitons which is then converted into electricity. An important limitation for solar cell efficiency is associated with spectral loss associated with the band gap of the material i.e; the chromophore will not be able to absorb photon below the energy band gap of the material and consequently this energy is lost. Up conversion process which convert low energy photons into high energy photons can be utilised to harvest those unused sub-threshold photons there by increasing the efficiency of the solar cell. In organic molecules this can be achieved by Triplet-Triplet Annihilation (TTA) in which two low energy triplet exciton are converted into a high energy singlet exciton which can be absorbed by the cell.
Another limitation associated with efficiency of solar cell is thermalization ie, both the absorption of high and low energy photons generates one electron hole pair and the excess energy associated with high energy photons is lost as heat. This limitation can be overcome by multi exciton generation process in organic molecules via singlet fission. Singlet fission (SF) involves the conversion of a high energy singlet exciton into two low energy triplet excitons. Singlet fission is actually the opposite process of Triplet-Triplet Annihilation.
Integrating both Singlet fission and Triplet-Triplet Annihilation in a solar cell can significantly increase its efficiency by reducing energy loss associated with both low and high energy end of the solar spectrum. But, the integration of TTA and SF into a solar cell is limited by the fact that a very few chromophores are currently known to exhibit these photophysical processes. Exploration of the vast chemical space by computational methods could accelerate the discovery of materials capable of undergoing TTA and SF. We utilise Time Dependent Density Functional Theory (TDDFT) and post Hartree- Fock Methods such as Complete Active Space Self Consistent Field Method (CASSCF) and Multi Reference Configuration Interaction Method (MRCI) to describe the excited state properties of computationally designed new chromophores.
Ab initio Quantum Mechanical Investigation to Elucidate Atmospheric Chemical Reactions
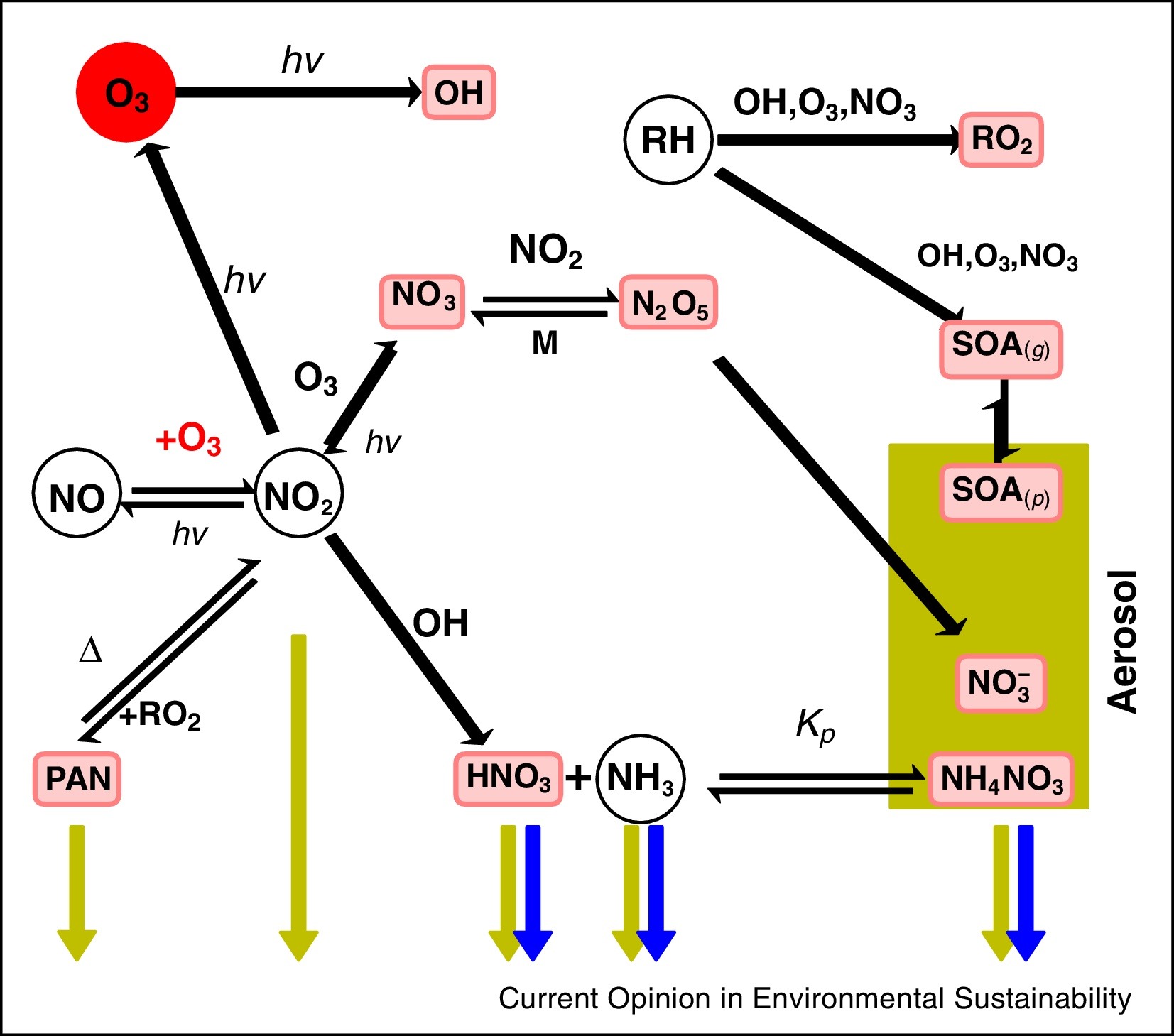
Atmospheric oxidative reactions are ubiquitous on our planet as these reactions orchestrate important effect on several environmental processes ranging from the formation of atmospheric contaminants to acid rain. The earth’s atmosphere especially ionosphere is a region of the upper atmosphere containing many ions. The most complicated chemistry occurs in the D-region and is noticeable for the existence of cluster ions. The composition of D-layers has important consequences for the attenuation of radio signal. Neutral species could be readily ionised by the interaction of the chemiions present in the atmosphere. Once these ions have been formed they can be involved in complicated reactions, and characterizing the mechanisms of these reactions is important for our understanding of the chemical composition of the atmosphere. These charged species could also be obtained from the combustion of fossil fuel in the jet engine exhaust. The combustion of fossil fuels in the jet engines as well as the rocket engine leads to the formation of major organic pollutants. Recent studies found that ozonation of organic pollutants with dimethylamino groups produces N-nitrosodimethylamine (NDMA) that is highly carcinogenic to humans. However, the formation mechanism of NDMA remains inexplicit, and previously proposed mechanisms are inconsistent with experimental observations. These atmospheric chemical phenomenons which involve the formation and removal of trace gases in the environment contribute to the determination of air quality.
Our focus in this area is to investigate the plausible mechanism of formation of the important gas phase nitrogenous compounds in the environment formed via the binary/ternary associative attachments between the nitrogen species with atmospheric trace gases. In particular we have been interested in developing different reaction pathways for the formation of negatively charged oxides of nitrogen, from the interaction of molecular nitrogen and oxides of nitrogen with the negatively charged oxygen ions(On-) and also the formation of neutral nitrosamines by the ozonation of different hydrazine compounds. For example we have recently developed the oxidative reaction pathway for the reaction of N2/NOx with superoxide and ozone anion. The nature of these reactions can be studied with quantum chemical calculations. Quantum chemical calculations have advantages in exploring reaction mechanisms by identifying transient species and providing thermodynamic data. We are also interested in developing the quantum chemical methods to study the structure, spectroscopy and reactivity of different positively and negatively charged hydrated cluster ion species in the atmosphere.
… and more.
